GCSE Chemistry breakdown: topics, learning objectives & sample questions

From atomic structure to organic reactions, GCSE Chemistry is packed with fascinating content and essential skills. This guide breaks down the key topics, learning goals and example questions to support confident revision and exam success.
What this post covers:
- Full topic list for GCSE Chemistry across AQA, Edexcel and OCR
- Key learning objectives for each topic
- Sample questions to support revision and exam practice
Why Chemistry matters for GCSE success
GCSE Chemistry is a core part of the Science curriculum. Whether taken as part of Combined Science or as a separate GCSE, it develops problem-solving, reasoning and applied maths skills that students carry into exams and beyond.
Mastering the content early gives students the confidence to tackle more challenging topics later on.
Exam boards and specification overview
GCSE Chemistry is assessed either as part of Combined Science or as a separate GCSE (Triple Science).
All major exam boards, AQA, Edexcel and OCR offer both routes, and the core content is broadly similar. However, there are key differences in depth, assessment, and grading.
Combined Science vs Triple Science
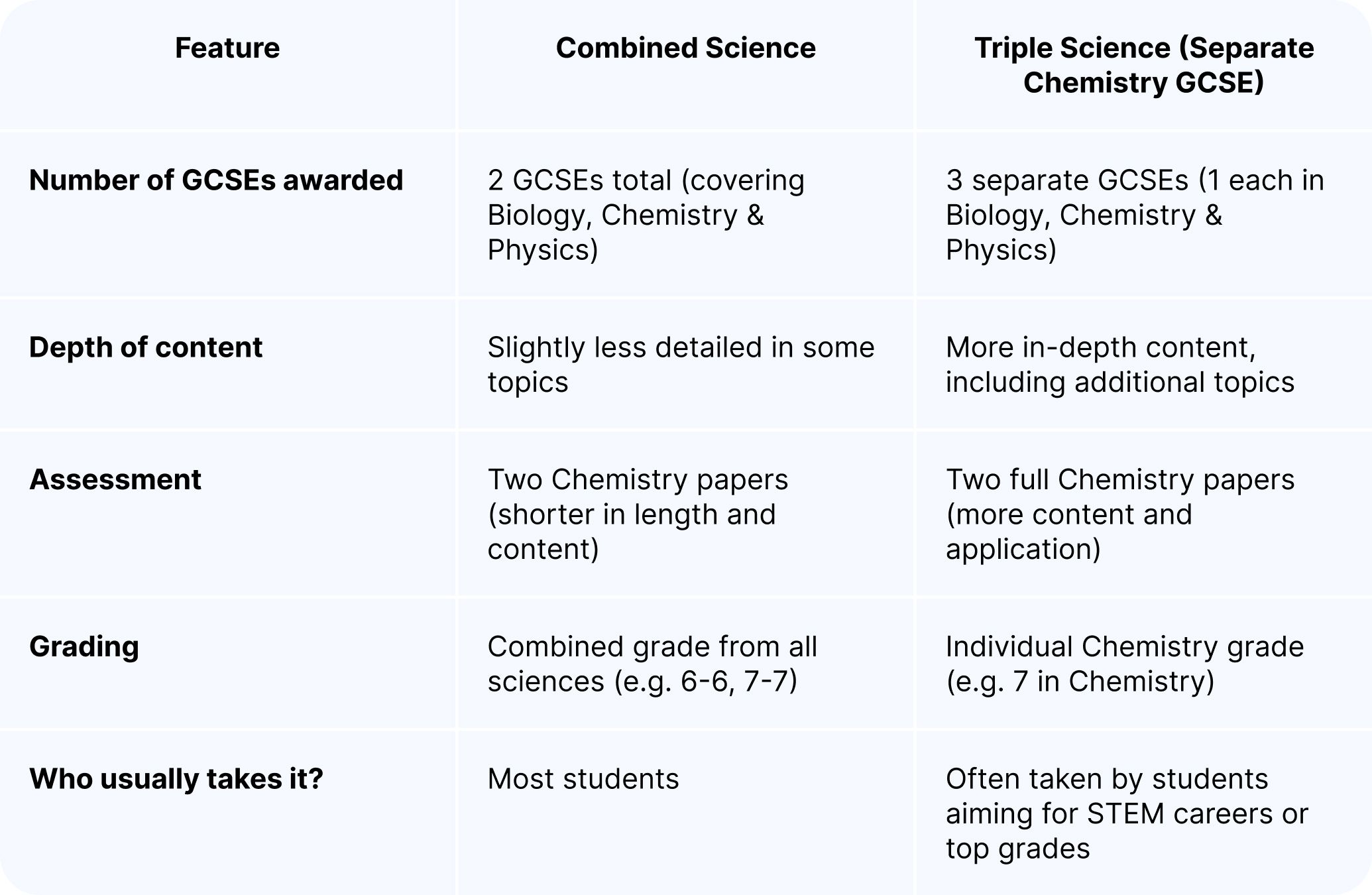
Combined Science students still study all the key Chemistry topics but with fewer examples and slightly reduced complexity.
Triple Science students may encounter more complex processes (e.g. chemical cells and fuel cells, or detailed organic synthesis pathways) and will have more questions requiring high-level application and analysis.
Exam board overview
While the themes are consistent, each exam board organises the content slightly differently:
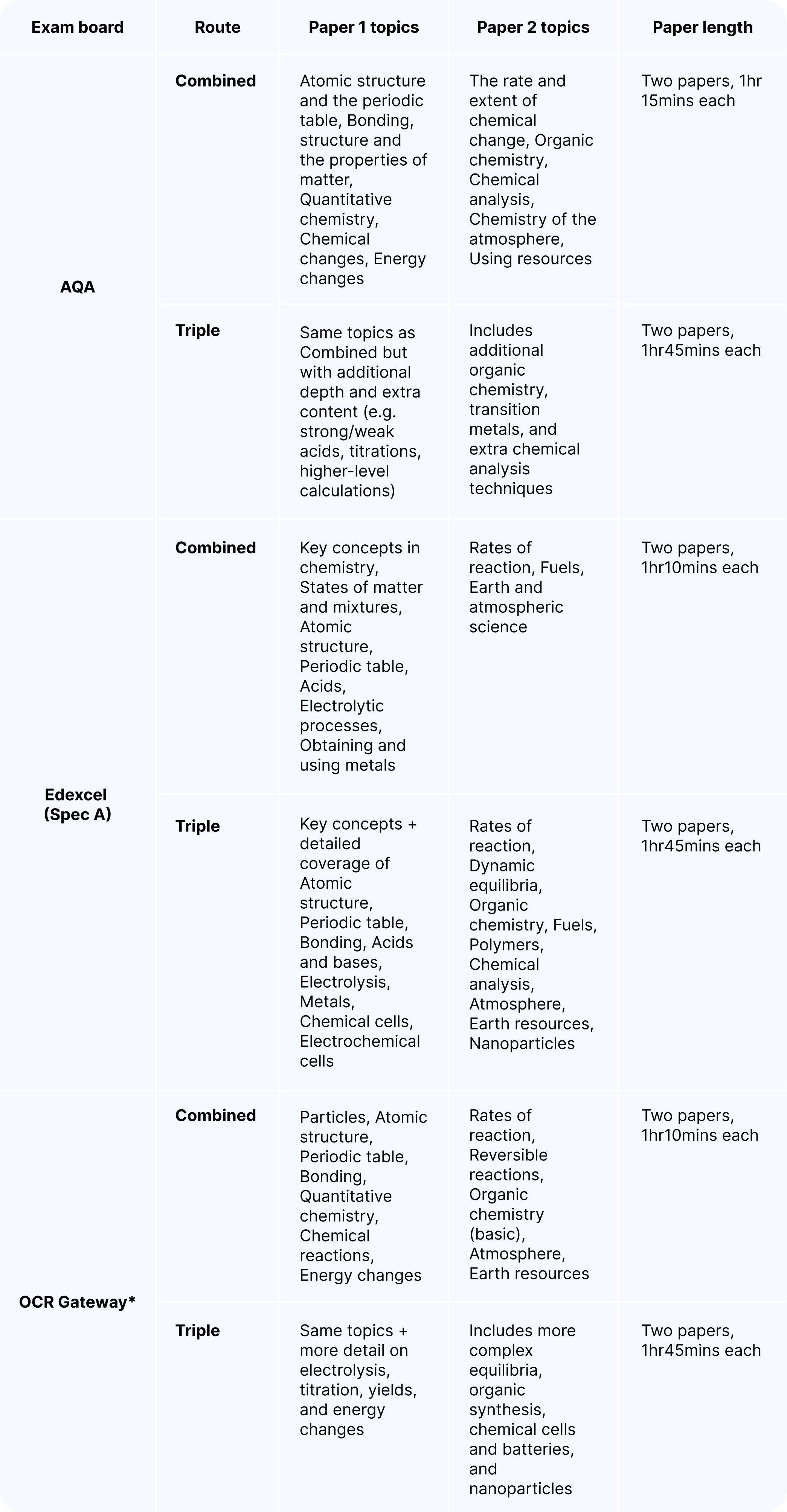
*OCR, offers more than one pathway (e.g. Chemistry A: Gateway Science and Chemistry B: Twenty-First Century Science). The core content is very similar, but topics may be grouped differently and assessed in slightly different ways. Make sure to check with your teacher which pathway your school is using.
Download your free GCSE revision planner
Less stress, more success! Get your free GCSE revision planner and our handy guide to effective revision today.

Chemistry key topics and learning objectives
1. Atomic structure and the periodic table
Learning objectives:
- Understand the structure of atoms, isotopes and electronic configurations
- Explain the development and layout of the periodic table
- Describe trends in reactivity and group properties
Sample question:
Explain why group 1 metals become more reactive as you go down the group (3 marks)
2. Bonding, structure and properties of matter
Learning objectives:
- Compare ionic, covalent and metallic bonding
- Link bonding and structure to physical properties
- Describe simple molecular, giant covalent and metallic structures
Sample question:
Describe how sodium and chlorine bond to form sodium chloride (4 marks)
3. Quantitative chemistry
Learning objectives:
- Calculate relative formula masses, moles and percentage composition
- Use the mole concept in equations
- Interpret limiting reactant and concentration problems
Sample question:
Calculate the number of moles in 25g of NaCl (Mr = 58.5) (2 marks)
4. Chemical changes
Learning objectives:
- Describe reactions of acids with metals, bases and carbonates
- Understand reactivity series and displacement reactions
- Prepare salts using laboratory methods
Sample question:
Explain how a pure, dry sample of copper sulfate can be made in the lab (6 marks)
5. Electrolysis
Learning objectives:
- Understand how electrolysis works and when it’s used
- Predict products of electrolysis of molten and aqueous solutions
Sample question:
Describe what is formed at each electrode when molten lead bromide is electrolysed (4 marks)
6. Energy changes
Learning objectives:
- Describe exothermic and endothermic reactions
- Interpret energy profile diagrams
- Understand bond energy calculations
Sample question:
Sketch and label an energy profile diagram for an exothermic reaction (3 marks)
7. Rate of reaction and equilibrium
Learning objectives:
- Explain collision theory and how conditions affect rate
- Analyse graphs showing rate changes
- Understand reversible reactions and dynamic equilibrium
Sample question:
Describe and explain the effect of temperature on the rate of a chemical reaction (6 marks)
8. Organic chemistry
Learning objectives:
- Recognise and describe alkanes, alkenes and polymers
- Understand cracking and combustion of hydrocarbons
- (Triple only) Learn reactions of alcohols, carboxylic acids and esters
Sample question:
Describe the test used to identify alkenes (2 marks)
9. Chemical analysis
Learning objectives:
- Interpret chromatograms and identify substances
- Understand tests for gases and ions
Sample question:
How can flame tests be used to identify metal ions? (3 marks)
10. Chemistry of the atmosphere
Learning objectives:
- Describe the evolution of the Earth’s atmosphere
- Explain the greenhouse effect and climate change
- Understand atmospheric pollutants and their sources
Sample question:
Describe how human activity has increased the amount of greenhouse gases in the atmosphere (4 marks)
11. Using Earth’s resources
Learning objectives:
- Understand finite and renewable resources
- Explain water purification and life cycle assessments
- Describe methods of metal extraction
Sample question:
Explain how potable water is produced from groundwater (4 marks)
Tips for revision and exam preparation
A strong revision plan for GCSE Chemistry balances understanding key concepts with regular practice.
Here are some strategies that make a real difference:
1. Break topics into manageable chunks
Chemistry can feel overwhelming when viewed as one long list of facts. Divide the syllabus into smaller sections, focusing on one topic, like bonding or rates of reaction, at a time. This helps build confidence and prevents revision fatigue.
2. Use active recall
Instead of re-reading notes, test knowledge regularly. This could be through flashcards, quick quizzes, or covering up answers and trying to recall key definitions or equations from memory. Active recall strengthens long-term memory far more effectively than passive revision.
3. Apply concepts to questions early
Don’t wait until the end of revision to attempt exam-style questions. Applying knowledge to real exam questions from past papers helps highlight gaps, improve exam technique, and make the content more memorable.
4. Practise the required practicals
Chemistry exams often test understanding of practical work, even without experimenting. Reviewing diagrams, method steps, and variables ensures students can confidently explain what’s happening and why.
5. Focus on tricky areas early
Topics like quantitative chemistry or organic reactions can take longer to master. Tackling these early leaves plenty of time to review and practise without the pressure of looming exams.
6. Make use of visual tools
Mind maps, diagrams, and flow charts are powerful for linking ideas, such as connecting the properties of materials to their bonding type. These can make complex topics easier to understand at a glance.
Final thoughts
With the right strategy and tools, GCSE Chemistry doesn’t need to be stressful. Focus on consistent revision, practise past questions, and remember, small steps lead to big gains.
Take control of your GCSE revision
Everything you need for GCSE success, in one place.
Feeling confident about your GCSEs comes from knowing what to revise, how to improve, and where to focus next.
🔮 GCSE practice papers based on our team's 2026 exam predictions
🎓 Guided courses that cover all the topics on your exams
✍️ Instant feedback that tells you where you’ve gained and lost marks
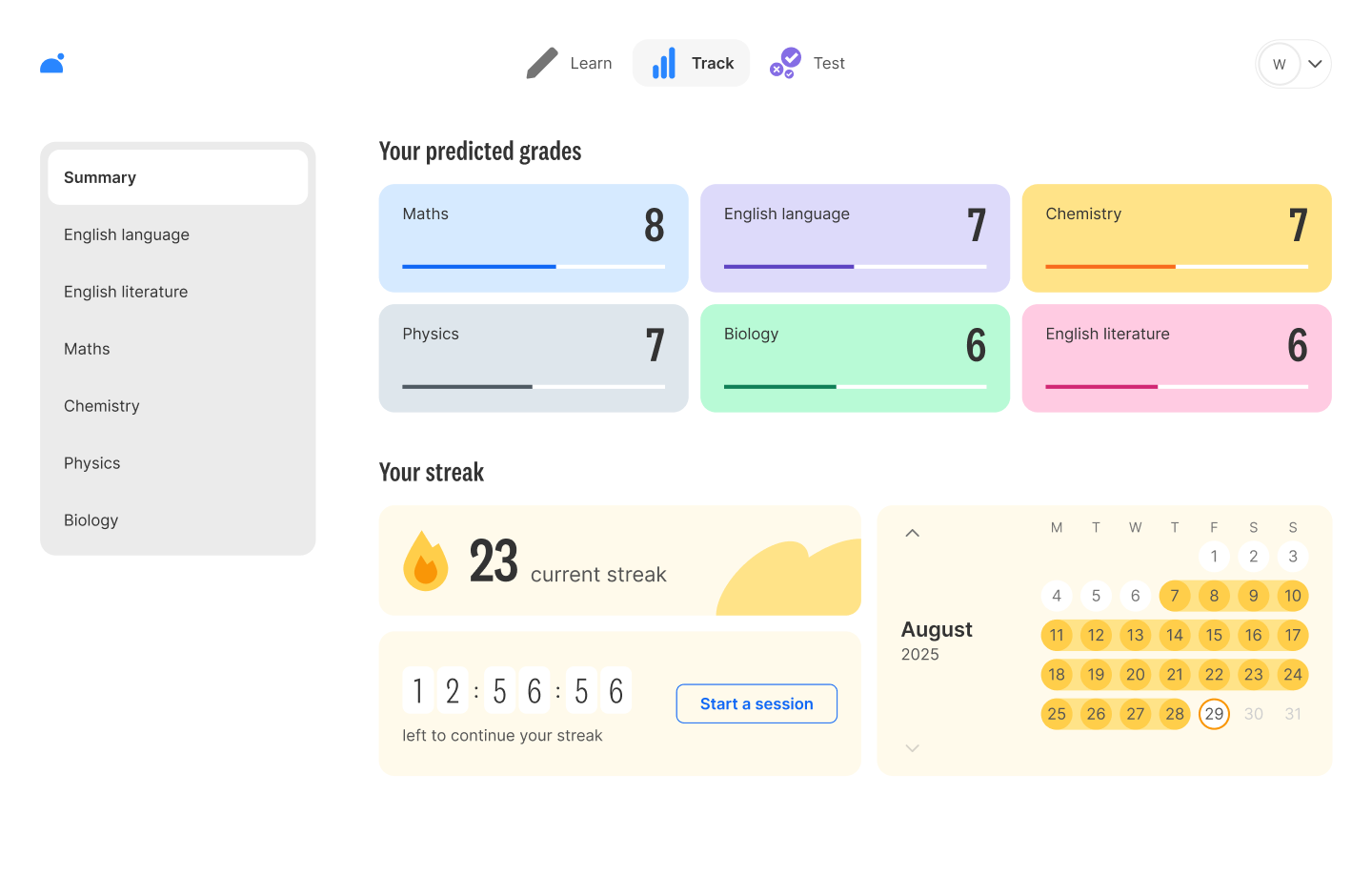
📈 Predicted GCSE grades and topic-by-topic tracking
Create your free account and start making real progress today.